Get 10% discount on first order
Challenge Test
Test the efficacy of your preservative system to ensure microbial safety of your product throughout its shelf life.

Easy process
We’ll guide you through the process
FREE PICKUP
We arrange the pick up of the samples

Customer care
You will be updated at every step on the way
Challenge Test
279.00€ – 299.00€
Objective
The objective of the challenge test, sometimes referred to also as the preservative efficacy test (PET test) is to check the effectiveness of the preservative system of the cosmetic product. Manufacturers of cosmetic products need to ensure that microorganisms introduced during normal product use through the exposure to the environment and from direct contact with the human skin, won’t negatively affect the safety or quality of cosmetic products. This is ensured by performing the challenge test.
Challenge Test
- MicroogranismsMicroogranisms
- Test timeTest time
- Sampling times in daysSampling times in days
- MicroogranismsEscherichia coli, Staphylococcus aureus,Pseudomonas aeruginosa, Candida albicans, Aspergillus brasiliensis
- Test time28 days
- Sampling times in days14 & 28
- MicroogranismsEscherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, Aspergillus brasiliensis
- Test time28 days
- Sampling times in days7, 14, 21 & 28
- MicroogranismsStaphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, Aspergillus brasiliensis
- Test time28 days
- Sampling times in days0, 2, 7, 14 & 28
- MicroogranismsStaphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, Aspergillus brasiliensis
- Test time28 days
- Sampling times in days7, 14 & 28
“I had such a good experience using them for our product testing! Tadej walked me through everything I needed to do and made it very simple. We have the results now and can use them to expand where we sell!”
“Tadej & his team were very knowledgeably in our testing and registration of products for eligible sale internationally. We continue to use them, and we highly recommend using this company for any of your testing needs.”
“That was way easier, cheaper, and more intuitive than expected,.. after ordering our tests, they've arranged a sample pickup and sent us results and documentation over the email. Recommended.”
“Very simple and fast way to test cosmetic products. I was satisfied with the service as everything went smoothly and in the faster time frame possible. I also got offers from some other laboratories and prices at Cosmetic Testing Lab were the most affordable.”
Customers
Challenge Test is required in the following countries:
Non-exhaustive list
Which products do or don’t require it?
Challenge test is required for all cosmetic products with the exception of microbiologically low-risk products, which are the following:
- Products that don’t contain water/have low water activity
- Products with a pH below 3 or above 10
- Products containing more than 20% alcohol
- Products containing raw materials that can create a hostile environment (strong oxidizing agents, polar organic solvents, oxidizing dyes, aluminium chlorohydrate and related salts, propellant gasses etc.)
- Products that are filled in containers at more than 65 degrees Celsius
- Products packaged in pressurized containers, pump dispensers or single-dose units
Which legislations require it?
- European Union and EEA: consisting of the 27 EU member states as well as Liechtenstein, Norway and Iceland
- Switzerland
- United Kingdom
- Turkey
- Serbia
- Gulf Cooperation Council countries: Saudi Arabia, Kuwait, Bahrain, Qatar, United Arab Emirates, Oman
- ASEAN countries: Indonesia, Thailand, Singapore, Malaysia, Philippines, Vietnam, Cambodia, Brunei, Myanmar, Laos
- South Africa
- New Zealand: it’s a standard to provide the challenge test, but it’s not mandatory
- Argentina
- Brazil
- Chile
- USA and Canada: safety of the product needs to be proven (not prescribed how), challenge test may be one way of doing it
Testing procedure
There is no universal standard for performing the challenge test, but all of the various testing protocols follow the same principles.
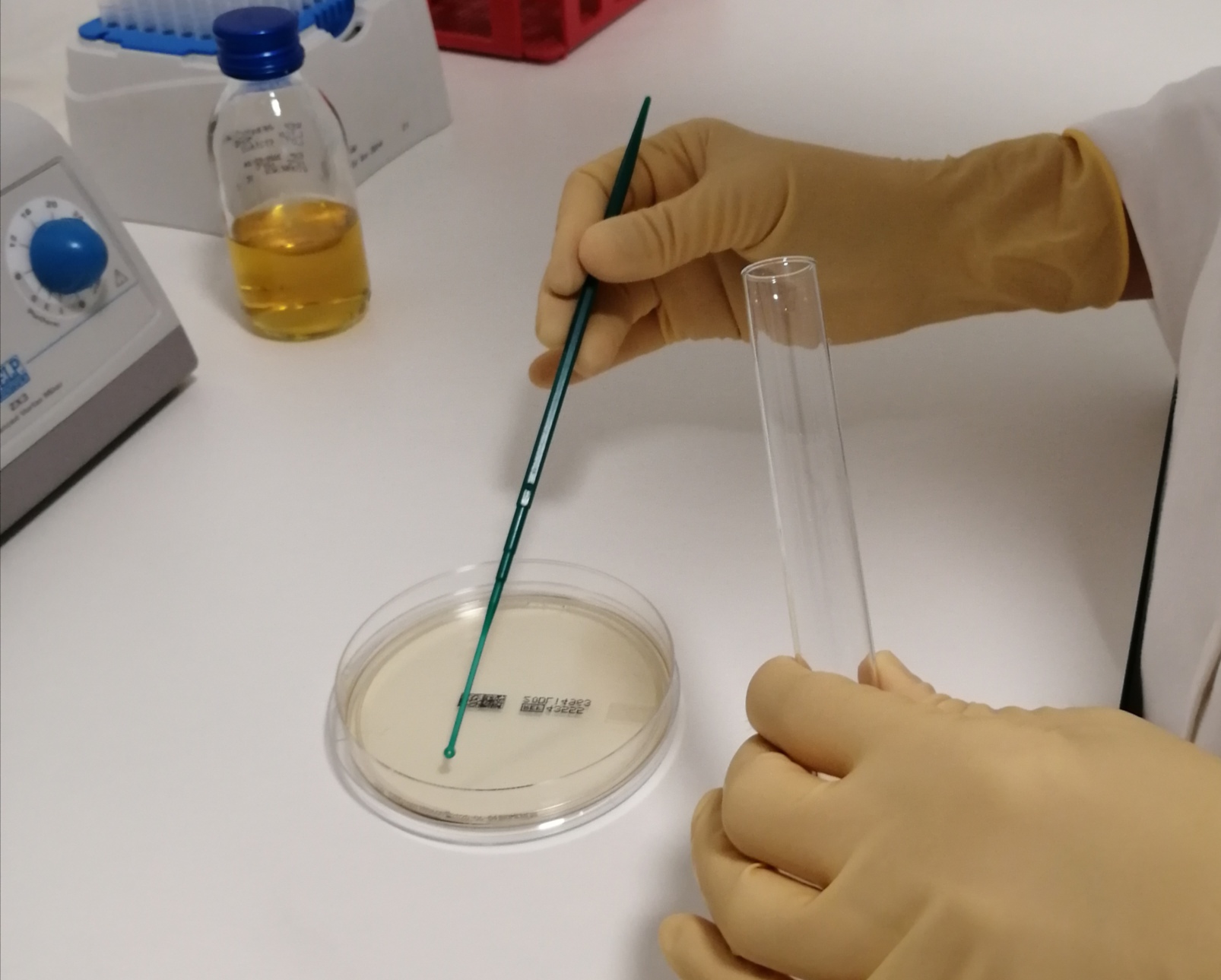
Cosmetic product samples are inoculated (artificially contaminated) with different strains of microorganisms, after which the evaluation of the decrease in contamination levels is monitored.
In order for the product to pass the testing, its preservation system has to be sufficiently effective to decrease the level of microorganisms to the allowed, predefined microbial limits. Microorganisms used in challenge testing are usually the following: Pseudomonas aeruginosa, Staphylococcus aeruginosa, Candida albicans and Aspergillus brasiliensis.

There are various standards for performing the challenge test, but there are only small differences between them.
We recommend the ISO 11930:2019 standard, but we can also offer the following:
- USP 51
- Japanese Pharmacopoeia
- European Pharmacopoeia / British Pharmacopoeia
- ISO 11930:2019
Timeline
Most of the challenge test standards require a 4 week – 28-day test.







Place an order
Find the tests that you need, fill in the necessary data, and press purchase
Prepare the samples
You will receive the information about how many samples we need for the requested tests
Samples shipping
On checkout you will select a date when our shipping partner will come to pickup the samples
Testing
Once we receive the samples, we will confirm the date when the testing will be completed and when you will receive the testing results
5
Testing results
When the tests are completed, we will send you the testing results via email